Lactobacillus plantarum 299v® (LP299v®) is one of the most researched strains from the L. plantarum species of bacteria. Bacteria from this species are rod-shaped and gram positive; they’re known to be highly adaptive and so are found in a wide variety of different sources, including vegetables, meat, fish, and dairy products. They also seem to be one of the best probiotic strains for use in cultured foods, and so are found in many fermented foods such as kimchi, sauerkraut, pickles, olives, and sourdough. Consequently, Lactobacillus plantarum strains are commonly found in the gastro-intestinal tract. Over the past decade or so, there has been an increasing number of studies which have attempted to identify the beneficial effects of numerous different L. plantarum strains on human health; however, the Lactobacillus plantarum 299v® strain is by far the most widely researched member of the species. As of April 2020 L. plantarum has been officially reclassified to Lactiplantibacillus plantarum subsp. plantarum so the full strain name may also be referred to as Lactiplantibacillus plantarum subsp. plantarum LP299v® (Zheng J et al., 2020).
Lactobacillus plantarum 299v® is a food supplement with research demonstrating its safety and survival to reach the gut alive. A commercial formula containing the strain was tested in individuals to assess safety in 2012 by Krag, A. et al., and on analysis of the results the authors deemed the strain to be safe and generally well tolerated. This was also confirmed in a previous study by Adawi, D et al., in 2002, who assessed the safety of the strain in an animal model.
In 2006, a study published by Goossens, D. et al., found L. plantarum 299v® to be present in stool samples and in some mucosal samples after oral administration indicating successful passage through the digestive tract. A previous study by Goossens, D. et al., (2005) also confirmed these results concluding the strain was able to reach the gut alive.

Irritable Bowel Syndrome is an increasingly common digestive disorder which encompasses a broad range of symptoms, including diarrhoea, constipation and bloating. Once any serious underlying causes have been ruled out, then medical professionals are limited in the options they can offer for management of the condition due to the variations in cause and symptoms, heterogeneity of this condition. The use of probiotics is being investigated as a useful addition to conventional solutions, and this is why the majority of strains featured in this database have been researched for IBS symptoms.
A double-blind, randomised, placebo controlled 4 week study investigated whether the introduction of a probiotic would affect symptoms of pain and bloating in 60 IBS patients. After 10 days of supplementation with the Lactobacillus plantarum 299v® strain, tests revealed that the probiotic had adhered to the gut lining and was still present 10 days post supplementation’ in 84% of the treatment group. The probiotic group also experienced a decrease in symptoms of flatulence. After four weeks of supplementation, twice as many patients from the probiotic group reported less flatulence than those in the placebo group, as well as experiencing less pain and more consistent bowel movements. This positive difference in results between the probiotic and placebo group was still evident twelve months later (Nobaek S. et al., 2000).
In a further study, a total of 214 IBS (of either sub-type) patients were recruited and randomly divided into two groups, a treatment group and a control group. Those in the treatment group were given a capsule containing the probiotic LP299v®, while those in the control group were given a placebo. Incidences of bowel frequency, abdominal pain, bloating and feelings of incomplete rectal emptying were all assessed weekly, and stool frequency was also monitored. Results showed that LP299v® helped with the symptoms of IBS, most particularly with pain and bloating (Ducrotté P. et al., 2012).
Other relevant studies: Niedzielin K. et al., (2001), Ribeiro HJ (1998), Sen S. et al., (2002), Simren M. et al., (2006), Stevenson C. et al., (2014), Young R., (1997).
Iron deficiency is an extremely prevalent and concerning nutritional deficiency that is becoming a major global health concern. It is a particularly common symptom in menstruating women. Both over-the-counter and prescription iron supplements are widely used, but even with supplementation, this additional iron may not be well-absorbed due to digestive insufficiencies and poor bio-availability. Unabsorbed iron in the gut is associated with constipation.
There has been some interest in the use of probiotics to help with iron deficiency, as lactic-acid fermented foods have been shown to help increase iron absorption via a series of different factors. This positive effect is believed to be due to the lactic acid preventing the formation of less bio-available iron molecules, and the presence of such organic acids delaying gastric emptying and exposing iron molecules to the intestinal lining for longer periods. Research on Lactobacillus plantarum 299v® suggests that this particular strain may be of particular benefit for improving the absorption of iron in the small intestine (Hoppe et al., 2015).
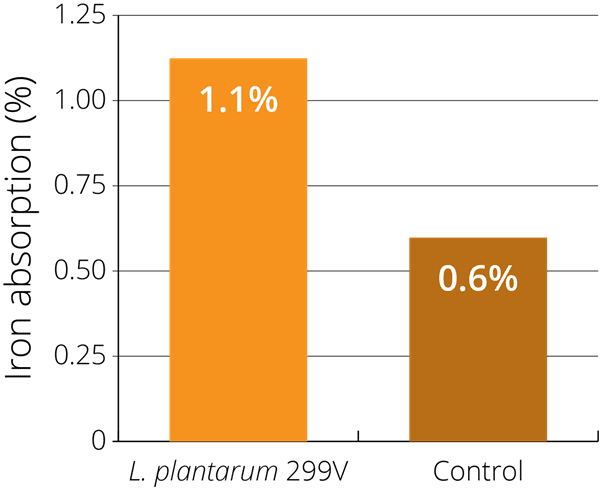
This potential was illustrated in a single blind, randomised study using 24 healthy women of childbearing age (with an average age of 25 years old). The women were given four different types of gruel-based meals over four consecutive days: fermented gruel containing live LP299v®, pasteurised fermented gruel, pH-adjusted non-fermented gruel, and non-fermented gruel with added organic acids. Iron absorption was measured 14–18 days after intake. It was found that, after consuming a meal with high amounts of phytic acid (which inhibits absorption), LP299v® increased iron absorption by 80% in comparison with the other meals. The researchers therefore confirmed that LP299v® alone may increase iron absorption (Bering S. et al., 2006).
Other Relevant Studies: Bering S. et al., (2007), Hoppe et al., (2015), Johansson et al., (1993).
Inflammatory Bowel Disease (IBD) is a collective term referring to a group of bowel disorders where inflammation level is a key marker. The two primary conditions in this group are ulcerative colitis and Crohn's disease, both of which are characterised by intestinal inflammation, and cause a variety of other symptoms such as diarrhoea, abdominal cramping, fatigue, fever and weight loss. It is now widely accepted that the balance of intestinal bacterial flora contributes significantly to the pathogenesis of inflammatory bowel disease, and so probiotics are being considered as a possible supportive supplement.
With the aim of exploring the impact of probiotic supplementation on the symptoms of IBD, an uncontrolled pilot study in Copenhagen observed 39 patients with mild to moderate active ulcerative colitis. All patients received a fermented oat drink containing LP299v® twice daily for 6 months. At the end of the study period, it was found that 24 out of the 39 taking the probiotic drink reported a 50% reduction their SSCAI (Simple Clinical Colitis Activity Index) score (Krag et al., 2012).
Building on these results, the study authors conducted a randomised controlled trial using 74 patients who were experiencing a mild to moderate flare up of their ulcerative colitis symptoms. The patients were randomly divided into two groups, one in which the patients received a fermented oat drink containing LP299v®, and another group in which the subjects received an energy drink containing protein and vitamins, which is often given to IBD patients. After 8 weeks of treatment, the mean reduction (as defined by the SSCAI score) in IBD symptoms was higher in the probiotic group, with over 50% of the patients showing an improvement (Krag A. et al., 2013).
Further Related Studies: Berggren A. et al., (2006), Jones C. et al., (2013), Lönnermark E. et al., (2015), McNaught C.E. et al., (2002), Wullt M., (2003).
Lactobacillus plantarum 299v® has been shown to have exceptional adherence and colonisation abilities to mucosal surfaces, even in the presence of antibiotics. A healthy presence of good bacteria helps to prevent pathogenic bacteria colonising in the intestine, which can often be a problem when taking antibiotics. The antibiotic medication can wipe out populations of beneficial microorganisms and allow pathogenic species such as Clostridium difficile to overgrow, causing associated diarrhoea symptoms. LP299v® has been shown to help alleviate antibiotic-associated diarrhoea (AAD), caused by Clostridium difficile infection, and helps to combat the proliferation of pathogenic bacteria.
A double-blind study observed 44 critically ill patients with an aim of assessing the positive benefits of the probiotic on the incidence of C. difficile infection. The subjects were randomised to be given either dose of LP299v® or a placebo. In the placebo group there was a 19% incidence of C. difficile infection, compared to the probiotic group, in which none of the patients contracted C. difficile infection (Klarin et al., 2008).
A further placebo-controlled study using 163 patients investigated the effect of LP299v® given both during a course of antibiotics and for a period after the course of medication. The overall risk of developing loose or watery stools, or nausea was significantly reduced among patients receiving the probiotic drink, compared to the placebo group. The results indicate that intake of L. plantarum 299v® may have a preventive effect on gastrointestinal symptoms during antibiotic treatment (Loennermark et al., 2010).
Other Relevant Studies: Kujawa-Szewieczek A., (2015), Levy J. (1997), Mack D.R. et al., (1997), Wullt M. et al., (2007).
There have been some promising preliminary studies which have explored the use of probiotics to reduce mucosal inflammation. It was found that when LP299v® adhered to the mucosal membrane of the intestine wall, the numbers of gram negative bacteria (typically pathogenic) reduced substantially. This was a significant finding, as such bacteria contain endotoxins, and even a small number can cause an extreme inflammatory reaction. This effect is thought to down-regulate the hosts ‘immunological defence’. Further research is required (Molin G., 2001).
Further Related Studies: Cunningham-Rundles S. et al., (2000), Cunningham-Rundles S. et al., (2011), McCracken et al., 2002), McNaught et al., (2005), Woodcock N.P. et al., (2004).
Authors: Information on this strain was gathered by Joanna Scott-Lutyens BA (hons), DipION, Nutritional Therapist; and Kerry Beeson, BSc (Nut.Med) Nutritional Therapist.
Last updated - 22nd May 2020
As some properties & benefits of probiotics may be strain-specific, this database provides even more detailed information at strain level. Read more about the strains that we have included from this genus below.
Lactobacillus acidophilus strains: Lactobacillus acidophilus LA-05, Lactobacillus acidophilus NCFM®, Lactobacillus acidophilus Rosell-52.
Lactobacillus casei strains: Lactobacillus casei Shirota, Lactobacillus casei DN-114001.
Lactobacillus plantarum strains: Lactobacillus plantarum LP299v.
Lactobacillus reuteri strains: Lactobacillus reuteri Protectis and Lactobacillus reuteri RC-14®.
Lactobacillus rhamnosus strains: Lactobacillus rhamnosus LGG®, Lactobacillus rhamnosus HN001, Lactobacillus rhamnosus GR-1® and Lactobacillus rhamnosus Rosell-11.
Lactobacillus paracasei strains: Lactobacillus paracasei CASEI 431®.
For more information and the latest research on probiotics, please visit the Probiotic Professionals pages.
Adawi, D. et al., (2002) ‘Safety of the Probiotic Strain Lactobacillus plantarum DSM 9843 (=strain 299v) in an Endocarditis Animal Model’. Microbial Ecology in Health and Disease, 14(1): 50-53. DOI: 10.1080/089106002760002766.
Adlerberth I. et al., (1996), ‘A mannose-specific adherence mechanism in Lactobacillus plantarum conferring binding to the human colonic cell line HT-29’. Appl Environ Microbiol 62: 2244-2251.
Bengmark S. & Gianotti L., (1996), ‘Nutritional Support to Prevent and Treat Multiple Organ Failure’. World J. Surg., 20:474–481.
Bering S. et al., (2006), ‘A lactic acid-fermented oat gruel increases non-haem iron absorption from a phytate-rich meal in healthy women of childbearing age’. British Journal of Nutrition, 96:80-85.
Bering S. et al., (2007), ‘Viable, lyophilized lactobacilli do not increase iron absorption from a lactic acid-fermented meal in healthy young women, and no iron absorption occurs in the distal intestine’. Br J Nutr., 98:991-997.
Berggren A. et al., (2006), ‘Intestinal function, microflora and nutrient intake of children after administration of a fermented oat product containing Lactobacillus plantarum DSM 9843 (299v)’. Helix Review Series: Pediatrics, 9:1-10.
Bukowska H. et al., (1998), ‘Decrease in fibrinogen and LDL-cholesterol levels upon supplementation of diet with Lactobacillus plantarum in subjects with moderately elevated cholesterol’. Atherosclerosis, 137: 437-438.
Cunningham-Rundles S. et al., (2002), ‘Development of Immunocompetence: Role of Micronutrients and Microorganisms’. Nutrition Reviews, 60(5):S68-S72.
Cunningham-Rundles S. et al., (2000), ‘Probiotics and immune response’. Am J Gastroenterol., 95:S22-25.
Cunningham-Rundles S. et al., (2011), ‘Effect of probiotic bacteria on microbial host defense, growth, and immune function in human immunodeficiency virus type-1 infection’. Nutrients, 3(12):1042-70.
Ducrotté P. et al., (2012). ‘Clinical trial: Lactobacillus plantarum 299v (DSM 9843) improves symptoms of irritable bowel syndrome’. World Journal of Gastroenterology, 18:4012-4018.
Goossens D. et al., (2003), ‘The effect of Lactobacillus plantarum 299v on the bacterial composition and metabolic activity in faeces of healthy volunteers: a placebo-controlled study on the onset and duration of effects’. Aliment Pharmacol Ther.18:495-505.
Goossens D. et al., (2005), ‘Survival of the probiotic, L. plantarum 299v and its effects on the faecal bacterial flora, with and without gastric acid inhibition. Digestive and liver disease: Official Journal of the Italian Society of Gastroenterology and the Italian Association for the Study of the Liver, 37:44-50.
Goossens D.A. et al., (2006), ‘Bowel cleansing with subsequent intake of Lactobacillus plantarum 299v does not change the composition of the faecal flora’. Microbial Ecology in Health and Disease, 18:139-146.
Goossens D.A. et al., (2006), ‘The effect of a probiotic drink with Lactobacillus plantarum 299v on the bacterial composition in faeces and mucosal biopsies of rectum and ascending colon’. Aliment Pharmacol Ther., 23: 255-263.
Granfeldt Y.E. & Björck I.M., (2011), ‘A bilberry drink with fermented oatmeal decreases postprandial insulin demand in young healthy adults’. Nutrition Journal, 10: 57.
Hoppe et al., (2015), ‘Probiotic strain Lactobacillus plantarum 299v increases iron absorption from an iron-supplemented fruit drink: a double isotope cross over single-blind study in women of reproductive age’. British Journal of Nutrition, 114:1195-120.
Johansson et al., (1993), ‘Administration of different Lactobacillus strains in fermented oatmeal soup: In vivo colonization of human intestinal mucosa and effect on indigenous flora’. Appl. Environ. Microbiol., 59:15-20.
Johansson M.L. et al., (1998), ‘Survival of Lactobacillus plantarum DSM 9843 (299v), and effect on the short chain fatty acid content of faeces after ingestion of a rose-hip drink with fermented oats’. International Journal of Food Microbiology, 42(1-2):29-38.
Jones C. et al., (2013), ‘Modulation of gut barrier function in patients with obstructive jaundice using probiotic LP299v’. Eur. J. Gastroenterol. Hepatol., 25(12):1424-30.
Karlsson C. et al., (2010), ‘Probiotic therapy to men with incipient arteriosclerosis initiates increased bacterial diversity in colon: A randomized controlled trial’. Atherosclerosis, 208: 228–233.
Kingamkono R. et al., (1999), ‘Enteropathogenic bacteria in faecal swabs of young children fed on lactic acid-fermented cereal gruels’. Epidemiol. Infect., 122: 23-32.
Klarin B. et al., (2005), ‘Adhesion of the probiotic bacterium Lactobacillus plantarum 299v onto the gut mucosa in critically ill patients: a randomised open trial’. Crit Care, 9:R285-293.
Klarin B. et al., (2008), ‘Lactobacillus plantarum 299v reduces colonisation of Clostridium difficile in critically ill patients treated with antibiotics’ Acta Anaesthesiologica Scandinavica, 52:1096-1102.
Krag A. et al., (2012), ‘Safety and efficacy of Profermin® to induce remission in ulcerative colitis’. World Journal of Gastroenterology, 18(15):1773-1780.
Krag A. et al., (2013), ‘Profermin is efficacious in patients with active ulcerative colitis--a randomized controlled trial’. Inflamm Bowel Dis., 19(12):2584-92.
Kujawa-Szewieczek A., (2015), ‘The Effect of Lactobacillus plantarum 299v on the Incidence of Clostridium difficile Infection in High Risk Patients Treated with Antibiotics’. Nutrients, 7(12):10179-88.
Levy J., (1997), ‘Experience with a juice beverage containing live Lactobacillus plantarum (LP) 299v: a promising adjunct in the management of antibiotic-related and other GI disturbances’. Annual Meeting of American Gastroenterological Association and American Association for the Study of Liver Diseases. Washington DC, USA.
Lönnermark E. et al., (2010), ‘Intake of Lactobacillus plantarum reduces certain gastrointestinal symptoms during treatment with antibiotics’. J. Clin. Gastroenterol. 44:106-112.
Lönnermark E. et al., (2015), ‘Effects of probiotic intake and gender on non typhoid salmonella infection’. J. Clin. Gastroenterol., 49(2):116-23.
Mack D.R. et al., (1997), ‘Short Course Antibiotics With Probiotics for Clostridium for Clostridium Difficile Colitis in Immunocompromised Patients: 118’. Journal of Pediatric Gastroenterology and Nutrition, 25(4):471.
Mack D.R. et al., (2003), ‘Extracellular MUC3 mucin secretion follows adherence of Lactobacillus strains to intestinal epithelial cells in vitro’. Gut. 52(6):827-833.
Mack D.R. et al., (1999), ‘Probiotics inhibit enteropathogenic E.coli adherence in vitro by inducing intestinal mucin gene expression’. Am. J. Physiol., 276:G941-G950.
Molin G., (2001), ‘Probiotics in foods not containing milk or milk constituents, with special reference to Lactobacillus plantarum 299v’. The American Journal of Clinical Nutrition,73(2): 380S-385S.
McCracken V.J et al., (2002), ‘TNF-α sensitizes HT-29 colonic epithelial cells to intestinal lactobacilli’. Experimental Biology and Medicine, 227:665-670.
Mangell P. et al., (2012), ‘Lactobacillus plantarum 299v Does Not Reduce Enteric Bacteria or Bacterial Translocation in Patients Undergoing Colon Resection’. Dig. Dis. Sci., 57(7):1915-24.
McNaught C.E. et al., (2005), ‘A prospective randomised trial of probiotics in critically ill patients’. Clinical Nutrition, 24:211-219.
McNaught C.E., (2002), ‘A prospective randomised study of the probiotic Lactobacillus plantarum 299V on indices of gut barrier function in elective surgical patients’. Gut, 51:827-831.
Naruszewicz M. et al., (2002), ‘Effect of Lactobacillus plantarum 299v on cardiovascular disease risk factors in smokers’. Am.J. Clin. Nutr., 76:1249-1255.
Niedzielin K. et al., (2001), ‘A controlled, double-blind, randomized study on the efficacy of Lactobacillus plantarum 299v in patients with irritable bowel syndrome’. Eur.J. Gastroenterol. Hepatol., 13(10):1143-7.
Nobaek S. et al., (2000), ‘Alteration of intestinal microflora is associated with reduction in abdominal bloating and pain in patients with irritable bowel syndrome’, Am. J. Gastroenterol. 95:1231-1238.
Önning G. et al., (2003), ‘Influence of a drink containing different antioxidants and Lactobacillus plantarum 299v on plasma total antioxidant capacity, selenium status and faecal microbial flora’. Int.J. Food Sci. Nutr. 54:281-289.
Oudhuis G.J. et al., (2011) Probiotics versus antibiotic decontamination of the digestive tract: infection and mortality. Intensive Care Med 37: 110-117.
Rask C et al. (2013), ‘Differential effect on cell-mediated immunity in human volunteers after intake of different lactobacilli’. Clin. Exp. Immunol., 172(2):321-32.
Ribeiro H.J. & Vanderhoof J.A., (1998), ‘Reduction of Diarrheal Illness Following Administration of Lactobacillus plantarum 299v in a Day-care Facility’. Journal of Pediatric Gastroenterology and Nutrition, 26(5):561.
Sen S. et al., (2002), ‘Effect of Lactobacillus plantarum 299v on colonic fermentation and symptoms of irritable bowel syndrome’. Dig. Dis. Sci., 47:2615-2620.
Simren M. et al., (2006), ‘Effects of Lactobacillus plantarum 299v on symptoms and rectal sensitivity in patients with irritable bowel syndrome (IBS) - A randomized, double-blind controlled trial’. Gastroenterology. A600.
Stevenson C. et al., (2014), ‘Randomized clinical trial: effect of Lactobacillus plantarum 299 v on symptoms of irritable bowel syndrome’. Nutrition. 30(10):1151-7.
Stjernquist-Desatnik A. et al., (2000), ‘Persistence of Lactobacillus plantarum DSM 9843 on human tonsillar surface after oral administration in fermented oatmeal gruel. A pilot study’. Acta Otolaryngol Suppl. 543: 215-219.
Vanderhoof J.A., (1998), ‘Treatment strategies for small bowel bacterial overgrowth in short bowel syndrome’. J. Pediatr. Gastroenterol. Nutr. 27:155-160.
Woodcock N.P. et al., (2004), ‘An investigation into the effect of a probiotic on gut immune function in surgical patients’. Clin Nutr.,;23(5):1069-73.
Wullt M. et al., (2007), ‘Lactobacillus plantarum 299v enhances the concentrations of fecal short-chain fatty acids in patients with recurrent clostridium difficile-associated diarrhea’. Dig Dis Sci., 52(9):2082-6.
Wullt M. (2003), ‘Lactobacillus plantarum 299v for the treatment of recurrent Clostridium difficile-associated diarrhoea: a double-blind, placebo-controlled trial’. Scand. J. Infect. Dis., 35:365-367.
Young R., (1997), ‘Therapeutic Efficacy of Lactobacillus Plantarum 299V in Chronic Recurrent Abdominal Pain of Childhood: 93’. Journal of Pediatric Gastroenterology and Nutrition, 25(4): 465.
Zheng J, Wittouck S. et al., (2020) 'A taxonmonic note on the genus Lactobacillus: Description of 23 novel genera, emended description of the genus Lactobacillus Beijerinck 1901, and union of Lactobacillaceae and Leuconostocaceae'. Int.J.Syst.Evol.Microbiol, 70(4): 2782-2858. DOI: 10.1099/ijsem.0.004107