

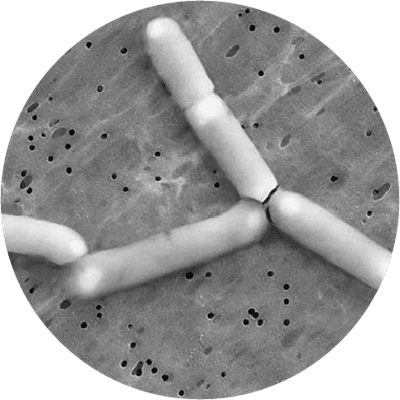
This strain is one of the most well documented probiotics for women’s intimate health, and fully deserves its place in this database of the world's best probiotic strains. It is a lactic acid forming bacteria which is rod shaped, gram positive, does not form spores and can move itself with flagella. It is best known for its ability to improve the balance of the vaginal microflora and has been extensively researched for this purpose, usually alongside the strain Lactobacillus reuteri RC-14®. The GR-1® strain is a member of the Lactobacillus rhamnosus species. As of April 2020 L. rhamnosus has been officially reclassified to Lacticaseibacillus rhamnosus so the full strain name may also be referred to as Lacticaseibacillus rhamnosus GR-1® (Zheng J et al., 2020).
The research conducted so far suggests that Lactobacillus rhamnosus GR-1® helps to protect against, and help manage infections of pathogenic bacteria or yeast in the female urogenital area, such as Bacterial Vaginosis, thrush and urinary tract infections (UTIs) which are also known as cystitis. It’s believed that the probiotic helps to alleviate and prevent the infections by adhering to the uretha, and has been shown in vaginal mucosa swabs to be present after oral administration. Though it may also adhere to the intestinal walls. As it competes for position, it displaces and prevents adhesion of pathogens to vaginal mucosa; however, it is primarily active in the vagina and urogenital area (Reid et al., 1995, Ewid et al., 2002).
In addition to the competitive inhibition of pathogens, as it fights for space in the mucosa, Lactobacillus rhamnosus GR-1® further discourages these harmful micro-organisms by the production of lactic acid which lowers the pH in the vagina; this is not conducive to the survival of pathogens as they tend to thrive in an alkaline environment. The probiotic also produces substance called a biosurfacant which further discourage pathogenic bacteria and yeasts, and inhibit their growth (Reid G. et al., 1998, McGroarty, 1998). These substances help to break down the biofilms created by pathogens, which can also help to make drug therapy more effective; these slimy biofilm barriers help to shield these undesirable micro-organisms from the action of drugs which would normally eradicate them (Lee, 2009, p351), (Saunders S. et al., 2007), (Reid et al., 2017).

Lactobacillus rhamnosus GR-1® is a food supplement which has been marketed around the world for a number of decades. Clinical trials have demonstrated its safety profile to be good, as would be expected from so many years of consumer use with no known cause for concern. Taking a look at one particular clinical trial that recorded incidence of adverse events, we see there is no difference between the probiotic and placebo groups. In this trial women took either L. rhamnosus GR-1® (in combination with Lactobacillus reuteri RC-14®) for 60 days. There was no difference in incidence of adverse events between the two groups, which demonstrates a satisfactory safety profile of this strain.
This strain has been detected in faecal samples and vaginal swabs following oral administration, which demonstrates good survival through the gut and as far as the vagina (Morelli, L. et al., 2004). In this trial faecal samples and vaginal swabs confirmed survival from 7 days after commencement of oral supplementation.
Bacterial Vaginosis (BV) is an imbalance in the populations of microflora in the vagina caused when two situations manifest. Firstly harmful bacteria, such as Gardnerella vaginalis, proliferate and cause infection and secondly, there is also a relative reduction in the presence of beneficial Lactobacilli. An overgrowth of such pathogens typically results in classic symptoms of a thin white/ grey discharge with a ‘fishy’ smell. The condition is extremely common, with as many as one in three women suffering from BV at some point in their lives (Shamshu R. et al., 2017). Conventional treatment for BV is in the form of antibiotics, but their effect is often only temporary and the rate of reoccurrence is high, so there is increasing scientific and medical interest in effective, natural solutions such as probiotics.
The potential benefits offered by probiotics were demonstrated in a randomised, placebo-controlled trial using 64 healthy women. The subjects were given daily oral capsules containing Lactobacillus rhamnosus GR-1® in combination with Lactobacillus fermentum RC-14® for two months. Following the trial, the authors released the following statement regarding the results:
‘Culture findings confirmed a significant increase in vaginal lactobacilli at day 28 and 60; a significant depletion in yeast at day 28 and a significant reduction in coliforms [typically harmful bacteria] at day 28, 60 and 90 for lactobacilli-treated subjects versus controls.’ (Reid et al., 2003).
In 2013, a further randomised, double-blind, placebo-controlled trial was set up to assess the efficacy of the bacterial strains, Lactobacillus rhamnosus GR-1® and Lactobacillus reuteri RC-14®, in alleviating BV symptoms. The trial monitored 544 otherwise healthy women, who were all over the age of 18 and had been diagnosed with bacterial vaginosis. The subjects were either given a placebo, or the probiotic capsules, for a period of six weeks. Vaginal swabs were taken at week six, then again at week twelve, and it was found that the vaginal microbiota had normalised in 243 (61.5%) of women in the probiotic group compared to only 40 (26.9%) of the women in the placebo group. This was recognised as a statistically significant result (Vujic et al., 2013).
A later study attempted to assess the effects of probiotic supplementation alongside antibiotics. The researchers recruited 62 women with BV, who were equally divided to receive either the probiotic supplement containing Lactobacillus reuteri RC-14® and L. rhamnosus GR-1®, or a placebo, whilst taking the antibiotic Tin****ole. Changes in the vaginal microflora were monitored using 16S rRNA gene sequencing both before and after treatment. It was found that Lactobacillus reuteri RC-14® and Lactobacillus rhamnosus GR-1® induced an increase in the relative abundance of indigenous flora in women with BV, helping to restore vaginal homeostasis (Macklaim et al., 2015).
The results of this study were substantiated further in a subsequent randomised, placebo- controlled trial using 32 women aged 18-45 years, who had all been diagnosed with BV. The women were divided into two equal groups, a treatment group in which participants were given a probiotic supplement containing Lactobacillus reuteri RC-14® and Lactobacillus rhamnosus GR-1®, and a control group in which all women were given a placebo. Both groups were also given a course of antibiotics alongside the supplements for the first seven days of the trial.
The probiotic and placebo groups both took two capsules of their respective supplements for the first 30 days of the trial, and then reduced this to 1 capsule a day for a further 30 days. To assess the results, a cervicovaginal smear was taken at the baseline, and then again at day 30 and at day 60. The results indicated that 81% of the probiotic group had a normal vaginal pH within 30 days of taking the probiotic, compared to only 31% of women in the placebo group (Shamshu R. et al., 2017).
Further relevant studies: Anukam et al., (2006), Burton et al., (2003), Cianci et al., (2008), Hummelen et al., (2010), Kamala et al., (2009), Krauss-Silva et al., (2011), Martinez et al., (2009), Perisić et al., (2011), Petricevic et al., (2008), Reid et al., 2001a), Reid et al., (2001b), Reid et al., (2003), Reid et al., (2004), Thulkar et al., (2010).
A study has shown that Lactobacillus rhamnosus GR-1® is effective in the prevention and treatment of urogenital infections (UTIs). UTIs, often called cystitis, are caused by pathogens such as Escherichia Coli colonising in the vagina, travelling up the urethra and finally infecting the bladder. To combat these infections, the mucosa carry specific receptors which, when stimulated by these gram negative pathogens, prompt the production of cytokines, and an immunomodulatory response is begun. It was found that Lactobacillus rhamnosus GR-1® supports this response and in doing so, can actually help to fight infection and reduce the incidence of UTIs (Beerepoot et al., 2016).
Another common problem with conventional antibiotic treatment for UTIs is the development of pathogenic antibiotic resistance, so there is a growing interest in the use of non-antibiotic solutions such as probiotics. In order to assess the potential of probiotics as an alternative to prophylactic antibiotic therapy, a double-blind, randomised clinical trial monitored 252 postmenopausal women with a history of recurrent UTIs or cystitis. The women were assigned to receive either the probiotic combination Lactobacillus reuteri RC-14® and Lactobacillus rhamnosus GR-1®, or antibiotics (trimethoprim-sulf******xazole) for one year. After the 12 month study period, the number of UTIs had more than halved in both groups, with the probiotics being almost as effective as antibiotics. Furthermore, after just one month in the antibiotic group, 90% antibiotic resistance had been developed to the prescribed medication, whereas the improvements had been made in the probiotic group without the risk of the pathogens becoming resistant (Beerepoot et al., 2012).
Further relevant studies: Bruce A.W. et al., (1988),Bruce A.W. (1992), Karlsson M. et al., (2012), Reid et al., (1992), Reid et al., (1995).
It is not just pathogenic bacteria that can overgrow and disrupt the balance of the vaginal microflora: vulvovaginal candidiasis (VVC), better known as vagina thrush, is caused by pathogenic yeasts, typically from the Candida family. Thrush infection is extremely common, second only to BV, and is believed to affect up to 75% of women at least once, although for many women, it is a recurrent issue that has detrimental effects on emotional and physical well-being (Martinez et al., 2009).
Thrush is a notoriously difficult condition to fully bring under control using conventional anti-fungal treatments such as flu*****ole and so, again, probiotics are being considered as a natural solution to support prescription and over-the-counter anti-fungal remedies. In order to try and demonstrate the efficacy of probiotics in this situation, 55 women were recruited, all of whom had been diagnosed with thrush and tested positive for Candida ssp. The women were given a probiotic supplement containing the strain Lactobacillus rhamnosus GR-1® together with Lactobacilli reuteri RC-14®, taken alongside an anti-fungal drug for a period of four weeks. Women assigned to the control group were given the anti-fungal treatment alone. The results showed that, after four weeks, there was a 70% reduction in thrush symptoms and yeast count in the probiotic group compared to the placebo group (Martinez et al., 2009).
Further relevant studies: Anukan et al., (2009).
The health of the vaginal microflora is known to be of vital importance in pregnancy, so there is considerable interest in the use of probiotics to maintain optimum intimate health at this important time. The vaginal ecosystem is very delicate and complex, lactobacilli are the main bacteria in this area in healthy women. A reduction in this genus of bacteria, particularly in the first trimester, can have serious effects and has even been found to increase the chances of pre-term labour; however, studies have indicated that the use of the probiotic Lactobacillus rhamnosus GR-1® may possibly reduce the risk of preterm labour (Gardiner et al., 2002). It’s believed that the anti-inflammatory effects of the probiotic may modulate various factors involved in the pathophysiology of preterm labour thereby, which could, in theory, reduce the risk of premature delivery (Yeganegi M., 2012).
It is known that babies receive their first influx of beneficial bacteria from their mother, so many new mothers are keen to ensure that their own microbiota is healthy. In a small trial using 56 pregnant women, who were given the probiotic supplement Lactobacillus rhamnosus GR-1® in combination with an extract from the Morgina plant, it was found that the combination appeared to increase the diversity of the gut bacteria of their new born babies (Bisanz J.E. et al, 2015).
Group B Streptococcus (GBS), or Streptococcus agalactiae, is a normal resident of the gut flora in 20-30% of people (both men and women), and may be found in the vaginal microflora in around 15-40% of all pregnant women. GBS is not usually associated with any health risks or symptoms, and most pregnant women who are known to host GBS go on to have healthy babies; however, there's a small risk that the bacteria can pass to the baby during childbirth and cause complications. If doctors believe that there is a risk of infection in a new baby, then they may prescribe antibiotics for mother and/or child (Ho et al., 2016). There is considerable interest in the potential of probiotics to reduce the incidence of GBS in pregnant women.
A randomised placebo-controlled trial was conducted to ascertain the effect of Lactobacillus rhamnosus GR-1® and Lactobacillus reuteri RC-14® on group B strep positive pregnant women. A total of 100 women testing positive for GBS were randomly assigned to be part of either a treatment group, in which members were given the probiotic combination, or a control group in which the participants were given a placebo. It was found that the GBS colonization changed from positive to negative in 42.9% of the probiotic group and just 18% in the placebo group. The authors concluded that these two strains of probiotic bacteria could therefore reduce the vaginal and rectal rate of colonization in pregnant women (Ho et al., 2016).
Further relevant studies: Kamala et al. (2009), Krauss-Silva et al., (2011).
Inflammation occurs naturally as part of the body’s immune response to foreign substances entering the body, or as part of the complex healing process which is triggered after trauma to a body part. However, associated health issues occur when these inflammatory responses are prolonged or inappropriate, perhaps being misdirected to benign substances or even the body’s own tissues. The role of probiotics to help modulate these inappropriate inflammatory responses is a developing area in the field of probiotic research.
Lactobacillus reuteri RC-14® and Lactobacillus rhamnosus GR-1® were tested to assess their anti-inflammatory potential in patients suffering from Inflammatory Bowel Disease (IBD). For the purposes of the trial, 20 healthy controls were sought, to compare with 20 patients who were suffering from IBD; fifteen of the IBD group were suffering from Crohn’s disease, and five were suffering from ulcerative colitis. All of the subjects were given yoghurt containing the probiotic combination for the test period of one month. It was found that the use of the probiotic promoted the formation of a desirable anti-inflammatory environment in the peripheral blood of IBD patients, and showed no harmful effects in these patients or control subjects. This effect was associated with an increase in the presence of T cells, with fewer such effects being observed in the healthy control group.
The positive effects of the probiotic yoghurt were confirmed by a follow-up study, in which subjects consumed the plain, non-probiotic yoghurt and did not experience the same benefits. These subsequent findings with plain, uncultured yoghurt indicate that the anti-inflammatory effects noted in the original study were dependent upon the presence of the Lactobacillus probiotic strains GR-1® and RC-14® (Baroja, 2007).
Further relevant studies: de los Angeles Pineda M.et al., (2011).
Authors: Information on this strain was gathered by Joanna Scott-Lutyens BA (hons), DipION, Nutritional Therapist; and Kerry Beeson, BSc (Nut.Med) Nutritional Therapist.
Last updated - 14th May 2020
As some properties & benefits of probiotics may be strain-specific, this database provides even more detailed information at strain level. Read more about the strains that we have included from this genus below.
Lactobacillus acidophilus strains: Lactobacillus acidophilus LA-05, Lactobacillus acidophilus NCFM®, Lactobacillus acidophilus Rosell-52.
Lactobacillus casei strains: Lactobacillus casei Shirota, Lactobacillus casei DN-114001.
Lactobacillus plantarum strains: Lactobacillus plantarum LP299v.
Lactobacillus reuteri strains: Lactobacillus reuteri Protectis and Lactobacillus reuteri RC-14®.
Lactobacillus rhamnosus strains: Lactobacillus rhamnosus LGG®, Lactobacillus rhamnosus Rosell-11 and Lactobacillus rhamnosus HN001
Lactobacillus paracasei strains: Lactobacillus paracasei CASEI 431®.
For more information and the latest research on probiotics, please visit the Probiotic Professionals pages.
Anukam et al., (2006), ‘Clinical study comparing probiotic Lactobacillus GR-1® and RC-14® with met*******ole vaginal gel to treat symptomatic bacterial vaginosis’. Microbes Infect.8(12-13):2772-6.
Anukam et al., (2009), ‘Oral use of probiotics as an adjunctive therapy to fluconazole in the treatment of yeast vaginitis: A study of Nigerian women in an outdoor clinic’. Microb. Ecol. Health Dis., 21(2):72-77.
Beerepoot et al., (2012), ‘Lactobacilli vs antibiotics to prevent urinary tract infections: a randomized, double-blind, noninferiority trial in postmenopausal women’. Arch. Intern. Med., 172(9):704-12.
Beerepoot M, Geerlings S. (2016) ‘Non-Antibiotic Prophylaxis for Urinary Tract Infections’. Young LS,
Svanborg C, eds. Pathogens. 2016;5(2):36. Bisanz et al., (2014), ‘Randomized open-label pilot study of the influence of probiotics and the gut microbiome on toxic metal levels in Tanzanian pregnant women and school children’. MBio, 5(5): (This trial was conducted using L. rhamnosus GR-1® only).
Bisanz J.E. et al., (2014), ‘A systems biology approach investigating the effect of probiotics on the vaginal microbiome and host responses in a double blind, placebo-controlled clinical trial of post-menopausal women’. PLoS One, 9(8):e104511.
Bisanz J.E. et al., (2015), ‘Microbiota at Multiple Body Sites during Pregnancy in a Rural Tanzanian Population and Effects of Moringa-Supplemented Probiotic Yogurt’. Appl. Environ. Microbiol., 81(15):4965-75.
Bruce A.W., & G. Reid, (1988), ‘Intravaginal instillation of Lactobacilli for prevention of recurrent urinary tract infections’. Can. J. Microbiol., 34:339-343.
Bruce A.W. et al., (1992), ‘Preliminary study on the prevention of recurrent urinary tract infections in ten adult women using intravaginal lactobacilli’. Int. Urogynecol. 3(1):22–25.
Burton et al., (2003), ‘Improved Understanding of the Bacterial Vaginal Microbiota of Women before and after Probiotic Instillation’. Appl Environ Microbiol; 69(1):97–101.
Cadieux P. et al., (2002), ‘Lactobacillus strains and vaginal ecology’ JAMA,287(15):1940-1.
Cianci et al., (2008), ‘Efficacy of Lactobacillus rhamnosus GR-1® and of Lactobacillus reuteri RC-14® in the treatment and prevention of vaginosis and bacterial vaginitis relapses’. Minerva Ginecol.,; 60(5):369-76.
de los Angeles
Pineda M. et al., (2011), ‘A randomized, double-blinded, placebo-controlled pilot study of probiotics in active rheumatoid arthritis’. Med. Sci. Monit; 17(6):CR347-CR354.
Gardiner G. et al., (2002), ‘Persistence of Lactobacillus fermentum RC-14® and Lactobacillus rhamnosus GR-1® but not L. rhamnosus GG in the Human Vagina as Demonstrated by Randomly Amplified Polymorphic DNA’. Clin, Vaccine Immunol, 9(1): 92-96.
Gardiner E. et al., (2002), ‘Oral administration of the probiotic combination Lactobacillus rhamnosus GR-1® and L. fermentum RC-14® for human intestinal applications’. International Dairy Journal, 12(2–3):191–196.
Ho M. et al., (2016), ‘Oral Lactobacillus rhamnosus GR-1® and Lactobacillus reuteri RC-14® to reduce Group B Streptococcus colonization in pregnant women: A randomized controlled trial’. Taiwanese Journal of Obstetrics & Gynaecology, 55:515-518.
Hummelen et al., (2010), ‘Lactobacillus rhamnosus GR-1® and L. reuteri RC-14® to prevent or cure bacterial vaginosis among women with HIV’. Int. J. Gynaecol. Obstet., 111(3): 245-8.
Hummelen et al., (2011), ‘Effect of 25 weeks probiotic supplementation on immune function of HIV patients’. Gut microbes; 2(2): 80-85.
Hummelen et al., (2011), ‘Effect of Micronutrient and Probiotic Fortified Yoghurt on Immune-Function of Anti-Retroviral Therapy Naive HIV Patients’. Nutrients; 10: 897-909. Note: This trial was conducted using L. rhamnosus GR-1® only
Irvine et al., (2010), ‘Probiotic Yoghurt consumption is associated with an increase of CD4 count among people living with HIV/ AIDS’. J Clin Gastroenterol.,44: 201. Note: This trial was conducted using L. rhamnosus GR-1® only.
Kamala et al., (2009), ‘Benefits of probiotic treatment in cases of bad obstetric history (BOH) and for prevention of post IVF pregnancy complications’. J. Obstet. Gynecol. India; 59(4): 336-339.
Karlsson M. et al., (2012), ‘Lactobacillus rhamnosus GR-1® enhances NF-kappa B activation in Escherichia coli-stimulated urinary bladder cells through TLR4’ BMC Microbiology, 12:15.
Krauss-Silva et al., (2011), ‘A randomised controlled trial of probiotics for the prevention of spontaneous preterm delivery associated with bacterial vaginosis: preliminary results’. Trials; 8(12): 239.
Koyama et al., (2010), ‘Development and pilot evaluation of a novel probiotic mixture for the management of seasonal allergic rhinitis’. Can. J. Microbiol., 56:730-738. Note: This trial was conducted using L. rhamnosus GR-1® only.
Lee Y, Salminen S. (2009), Handbook of Probiotics and Prebiotics, 2nd edition, Hoboken: John Wiley & Sons.
Macklaim et al., (2015), ‘Changes in vaginal microbiota following antimicrobial and probiotic therapy’. Microbial Ecology in Health and Disease, 26:27799.
Martinez et al., (2009), ‘Improved treatment of vulvovaginal candidiasis with fluconazole plus probiotic Lactobacillus rhamnosus GR-1® and Lactobacillus reuteri RC-14®’. Lett. Appl. Microbiol., 48(3): 269-74.
McGroarty J.A. & Reid G., (1998), ‘Detection of a Lactobacillus substance that inhibits Escherichia coli’. Canadian Journal of Microbiology, 34(8):974-8.
Martinez et al., (2009), ‘Improved cure of bacterial vaginosis with single dose of tinidazole (2g), Lactobacillus rhamnosus GR-1®, and Lactobacillus reuteri RC-14®: a randomized, double-blind, placebo-controlled trial’. Can. J. Microbiol.,55(2):133-8.
Morelli L. et al., (2004), ‘Utilization of the intestinal tract as a delivery system for urogenital probiotics’. J. Clin. Gastroenterol., 38(6 Suppl):S107-10.
Perisić et al., (2011), ‘The influence of probiotics on the cervical malignancy diagnostics quality’. Vojnosanit Preg., 68(11): 956-60.
Petricevic et al., (2008), ‘Randomized, double-blind, placebo-controlled study of oral lactobacilli to improve the vaginal flora of postmenopausal women’. Eur. J. Obstet. Gynecol. Reproductive Biology, 141(1): 54-7.
Reid et al., (1988), ‘Intravaginal instillation of lactobacilli for prevention of recurrent urinary tract infections’. Can. J. Microbiol., 34: 339-343. Note: This trial was conducted using L. rhamnosus GR-1 only.
Reid G. et al., (1992), ‘Influence of three-day antimicrobial therapy and Lactobacillus vaginal suppositories on recurrence of urinary tract infections’. Clinical Therapy, 4:11-16.
Reid G. et al., (1995), ‘Instillation of Lactobacillus and stimulation of indigenous organisms to prevent recurrence of urinary tract infections’. Microecology Therapy, 23:32-45.
Reid G., Tieszer C., & Lam D., (1995), ‘Influence of lactobacilli on the adhesion of Staphylococcus aureus and Candida albicans to fibers and epithelial cells’. Journal of Industrial Microbiology, 15:248.
Reid et al., (1995), ‘Instillation of Lactobacillus and stimulation of indigenous organisms to prevent recurrence of urinary tract infections’. Microecol. Ther., 23: 2-45. Note: This trial was conducted using L. rhamnosus GR-1® only.
Reid G. et al., (1998), ‘Lactobacillus inhibitor production against Escherichia coli and coaggregation ability with uropathogens’. Canadian Journal of Microbiology, 34(3):344-51.
Reid et al., (2001a), ‘Oral probiotics can resolve urogenital infections’. FEMS Immunol. Med. Microbiol., 30(1):49-52.
Reid et al., (2001b), ‘Probiotic Lactobacillus dose required to restore and maintain a normal vaginal flora’. FEMS Immuno.l Med. Microbiol., 32(1):37-41.
Reid et al., (2002), ‘Ability of Lactobacillus GR-1® and RC-14® to Stimulate Host Defences and Reduce Gut Translocation and Infectivity of Salmonella typhimurium’. The Korean Society of Food Science and Nutrition, 7(2):168-173.
Reid et al., (2003), ‘Oral use of Lactobacillus rhamnosus GR-1® and L. fermentum RC-14® significantly alters vaginal flora: randomized, placebo-controlled trial in 64 healthy women’. FEMS Immunol. Med. Microbiol., 35(2): 31-4. Note: L. fermentum RC-14 has since been re-classified as L. reuteri RC-14.
Reid et al., (2004), ‘Nucleic acid-based diagnosis of bacterial vaginosis and improved management using probiotic lactobacilli’. J. Med. Food, 7(2): 223-8.
Reid G. et al., (2017), ‘The development of probiotics for women’s health (review)’. Canadian Journal of Microbiology, 63:269–277.
Saunders S. et al., (2007), ‘Effect of Lactobacillus challenge on Gardnerella vaginalis biofilms Colloids and Surfaces B’. Biointerfaces, 2(1):138–142.
Shamshu, R., et al., (2017), ‘Role of probiotics in lower reproductive tract infection in women of age group 18 to 45 years’. International Journal of Reproduction, Contraception, Obstetrics and Gynecology, 6(2):671-681.
Thulkar et al., (2010), ‘Probiotic and met********ole treatment for recurrent bacterial vaginosis’. Int J Gynaecol Obstet; 108(3): 251-2.
Velraeds M. et al., (1996), ‘Inhibition of initial adhesion of uropathogenic Enterococcus faecalis by biosurfactants from Lactobacillus isolates’. Appl. Environ. Microbiol., 62(6):1958-1963.
Vujic et al., (2013), ‘Efficacy of orally applied probiotic capsules for bacterial vaginosis and other vaginal infections: a double-blind, randomized, placebo-controlled study’. Eur. J. Obstet. Gynecol. Reprod. Biol., 168(1): 75-9.
Yeganegi M. et al., (2011), ‘Lactobacillus rhamnosus GR-1® Stimulates Colony-Stimulating Factor 3 (Granulocyte) (CSF3) Output in Placental Trophoblast Cells in a Fetal Sex-Dependent Manner1’ Biol Reprod., 84(1): 8–25.
Zheng J, Wittouck S. et al., (2020) 'A taxonmonic note on the genus Lactobacillus: Description of 23 novel genera, emended description of the genus Lactobacillus Beijerinck 1901, and union of Lactobacillaceae and Leuconostocaceae'. Int.J.Syst.Evol.Microbiol, 70(4): 2782-2858. DOI: 10.1099/ijsem.0.004107