

Bacillus coagulans BC30™, also known as Bacillus coagulans GBI-30, 6086 or Bacillus coagulans Ganeden BC30™ is a bacterial strain belonging to the Bacillus coagulans species. B. coagulans BC30™ is a spore-forming, gram positive microbe that can survive in both aerobic and anaerobic conditions. It has a transient nature and is eliminated from the gastro intestinal (GI) tract four to five days after consumption (Donskey et al., 2001). The microbe is thought to germinate in the small intestine where it becomes biologically active, producing lactic acid, digestive enzymes and anti-microbials (Hyronimus et al., 2000; Jäger et al., 2018; Cao et al., 2020).
Researched in: children, adults and the elderly
B. coagulans BC30™ has been deemed safe for human consumption. An in vitro study assessed numerous toxicological parameters which confirmed the strain was safe and did not demonstrate any mutagenic, clasogenic, or genotoxic effects (Endres et al., 2009).
The strain has also been shown to be safe in several randomised clinical trials (RCT’s). They highlighted no significant differences in adverse events between probiotic and placebo groups (Dolin, 2009; Hun, 2009; Gepner et al., 2017).
An in vitro upper gastro intestinal tract model found B. coagulans BC30™ showed high survival rates through the stomach and reached the small intestine. Germination rates were lower than expected, however the researchers note this was likely due to conditions tested and may not represent germination levels in vivo (Maathuis, Keller and Farmer, 2010).
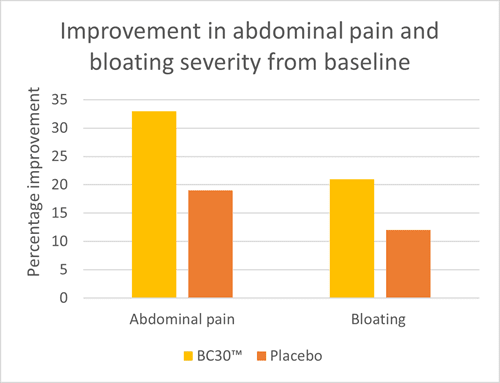
An RCT investigated the effect of B. coagulans BC30™ in 44 individuals with IBS-D confirmed by the Rome II IBS diagnostic criteria. Individuals were divided to receive either 0.8 billion CFU of B. coagulans BC30™ or placebo daily for eight weeks. Participants recorded severity of abdominal pain and bloating every day during the intervention period. At the end of the study, those in the probiotic group had significantly reduced abdominal pain and bloating. Weekly analysis suggested that the probiotic was effective in reducing abdominal pain and bloating after one week’s supplementation, with improved overall symptom scores compared to placebo (Graph 1). Placebo supplementation also resulted in significant reductions in abdominal pain at week six and week eight but no other weeks reached significance. Overall these results show potential for B. coagulans BC30™ to support those with IBS related abdominal pain and bloating (Hun, 2009).
A similarly designed RCT found B. coagulans BC30™ to significantly improve symptoms in participants with diagnosed IBS-D. The trial included 52 individuals who were divided to receive probiotic or placebo once a day for eight weeks. In the probiotic group, the average number of bowel movements significantly reduced compared to placebo, highlighting the strain’s ability to support healthy bowel movements (Dolin, 2009).
Another RCT assessed B. coagulans BC30™ in 61 individuals with self-reported GI issues. Participants were randomised to receive either a blend of 2 billion CFU of B. coagulans BC30™ and digestive enzymes or a placebo. They took their respective supplement daily for four weeks. In the probiotic group there were significant improvements in abdominal pain, total gastrointestinal symptom scores and a general improvement in abdominal distension (p=0.06) compared with the control groups. Researchers did note significant improvements in the placebo group which may explain why other end points like bloating and flatulence did not significantly improve in the therapeutic group. Overall the trial showed promise for the use of B. coagulans BC30™ in individuals with abdominal pain and distension (Kalman et al., 2009).
B. coagulans BC30™ may support gut health in older people, by encouraging the growth of other good bacteria which in turn offer positive benefits to the host. Levels of beneficial bacteria and short chain fatty acids (SCFA’s) often decline in older adults, which may account for the higher constipation rates and generally poorer immunity typically seen in this age group.
A crossover RCT assessed IBS symptoms in 36 elderly individuals (aged 65-80 years old). Participants were divided to receive one billion CFU of B. coagulans BC30™ or a placebo daily for 28 days. All individuals then underwent a 21 day wash out period before switching to the other treatment group for a further 28 days. When the probiotic was consumed, the researchers noted a significant increase in Faecalibacterium prausnitzii; a microbe that is receiving much scientific interest due to its high butyrate producing ability and anti-inflammatory nature. There was also a significant increase in the anti-inflammatory cytokine IL-10 when B. coagulans BC30™ was consumed. This study highlights exciting potential for the use of B. coagulans BC30™ in older people (Nyangale et al., 2015).
An RCT assessed upper respiratory tract infections (URTI’s) and gastrointestinal infections (GI) in 80 children aged 6 – 8 years. They were randomised to receive either one billion CFU of B. coagulans BC30™ or a placebo daily for three months. Children receiving B. coagulans BC30™ had significantly fewer respiratory symptoms (congested nose, itchy nose and hoarseness) and GI symptoms (flatulence) compared to placebo (Graph 2 and 3). Additionally, children in the probiotic group exhibited significantly fewer headaches and fatigue. Many other symptoms did seem to occur less frequently in the probiotic group; however, the results did not reach significance or trend (p<0.1).
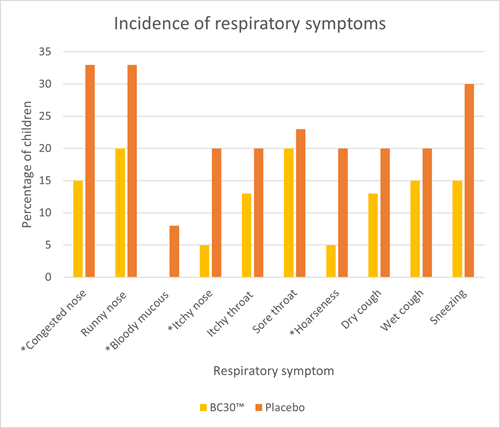
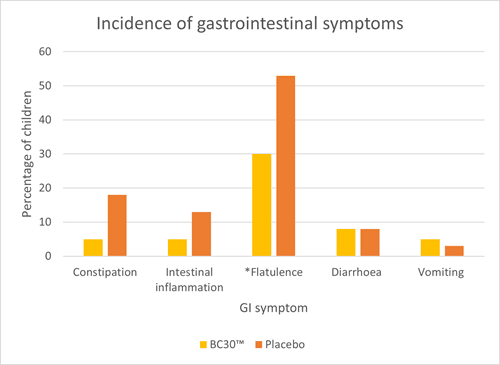
The study also noted significant reductions in inflammatory cytokines IL-6 and TNF- alpha in the probiotic group, suggesting a potential anti-inflammatory effect of B. coagulans BC30™. Overall, the study demonstrates encouraging results for the use of B. coagulans BC30™ in children. However, more studies are required in this age group to confirm results (Anaya-Loyola et al., 2019).
A small pilot study was conducted in 10 individuals to assess B. coagulans BC30™ during viral exposure. Participants consumed the probiotics for 28 days and immunological marker levels were assessed. Participants were exposed to adenovirus and influenza virus. Several immune markers were enhanced after viral exposure; CD3+CD69+ cells, IL-6, IL-8, interferon-gamma (IFN-gamma) and TNF-alpha. The study highlights some potential for B. coagulans BC30™ to boost immune responses, however more data is needed to elucidate the role and efficacy of B. coagulans BC30™ in immune health (Kimmel et al., 2010).
B. coagulans BC30™ has been shown to produce digestive enzymes including alkaline proteases. These enzymes may increase the presence of amino acids in the blood after protein intake (Levers et al., 2015). Additionally, the potential anti-inflammatory nature of B. coagulans BC30™ may support villi health, thus increasing nutrient absorption.
A randomised, double blinded and crossover study assessed B. coagulans BC30™ on protein absorption in 30 healthy adults. Individuals were divided to receive either 25g of milk protein concentrate plus one billion CFU of B. coagulans BC30™ or 25g of milk protein concentrate alone. They took their respective supplement daily for two weeks and then underwent a three-week washout period. Post washout, individuals swapped treatments and took the opposite intervention for a further two weeks. B. coagulans BC30™ consumption was associated with significantly higher blood concentrations of the amino acids arginine, serine, ornithine, methionine, glutamic acid, phenylalanine, isoleucine and tyrosine. The time to reach max concentration was increased for glutamine and alanine. The study offers promising results to individuals on low protein diets or with digestive and absorption issues (Stecker et al., 2020).
A validated in vitro upper GI model also found the addition of B. coagulans BC30™ to be associated with enhanced amino acid bioavailability (Keller et al., 2017). Whilst the probiotic increased protein availability in the upper parts of the small intestine, it was also able to reduce the amount of protein delivered to the colon (thus reducing fermentation into toxic metabolites and gas).
Limited research has been conducted into the use of probiotics to support with athletic performance; however, researchers have investigated this area with B. coagulans BC30™ and found encouraging results.
A study included 29 males with at least three months of recreational training. The study lasted seven weeks in total which included a wash out period. Individuals received 20g casein daily for the first two weeks and then underwent exercise and testing. After a one-week washout, participants then received 20g casein daily plus one billion CFU of B. coagulans BC30™ for two weeks followed by exercise and testing. B. coagulans BC30™ consumption resulted in:
Increased recovery at 24hrs and 72hrs post exercise (48hrs; p=0.06)
Reduced muscle soreness at 72hrs
No significant differences were noted in performance measures between probiotic and control supplementation. However, the use of the probiotic did help to maintain physical performance. Overall, the probiotic could be used by athletes and other exercise goers to reduce muscle soreness and increase recovery rates (Jäger et al., 2016).
A small pilot study including 10 men undergoing four body workouts per week found the use of B. coagulans BC30™ to moderately improve jump power (p=0.1) over an eight week study period (Georges et al., 2014).
A double blind, parallel design study recruited 25 male soldiers to participate in a study assessing the use of B. coagulans BC30™ with β-hydroxy-β-methylbutyrate (HMB) calcium (CaHMB) on inflammatory responses and muscle integrity. The soldiers were randomly divided to three groups. Group 1 received either one billion CFU of B. coagulans BC30™ alongside CaHMB (n=9). Group 2 received CaHMB and placebo (n=8) and Group 3 received no supplementation and acted as a control group (n=8). Each group took their respective supplement daily for 40 days. Training and exercises continued as normal throughout the study and similar for all soldiers, activities included combat skill development, krav maga, runs carrying heavy equipment, and navigational training. The study found Group 1 and Group 2 had significantly reduced levels of inflammatory cytokines; with group 1 exhibiting significantly reduced IL-6 levels. In addition, group 1 who took B. coagulans BC30™ were able to maintain muscle integrity compared to group 2. The results suggest B. coagulans BC30™ may augment the effects of CaHMB alone and could provide additional benefits such as supporting muscle integrity (Gepner et al., 2017).
Author: Information on this strain was gathered by Dr Kate Steed PhD Food and Microbial Sciences; Gut Microbiology (University of Reading), BSc Medical Microbiology.
For more information and the latest research on probiotics, please visit the Probiotic Professionals pages.
Anaya-Loyola, M. A. et al. (2019) ‘Bacillus coagulans GBI-30, 6068 decreases upper respiratory and gastrointestinal tract symptoms in healthy Mexican scholar-aged children by modulating immune-related proteins’, Food Research International, 125, p. 108567. doi: 10.1016/j.foodres.2019.108567.
Cao, J. et al. (2020) ‘Probiotic characteristics of Bacillus coagulans and associated implications for human health and diseases’, Journal of Functional Foods, 64. doi: 10.1016/j.jff.2019.103643.
Dolin, B. J. (2009) ‘Effects of a propietary Bacillus coagulans preparation on symptoms of diarrhea-predominant irritable bowel syndrome’, Methods and Findings in Experimental and Clinical Pharmacology, 31(10), p. 655. doi: 10.1358/mf.2009.31.10.1441078.
Donskey, C. J. et al. (2001) ‘Effect of oral Bacillus coagulans administration on the density of vancomycin-resistant enterococci in the stool of colonized mice’, Letters in Applied Microbiology, 33(1). doi: 10.1046/j.1472-765X.2001.00948.x.
Endres, J. R. et al. (2009) ‘Safety assessment of a proprietary preparation of a novel Probiotic, Bacillus coagulans, as a food ingredient’, Food and Chemical Toxicology, 47(6), pp. 1231–1238. doi: 10.1016/j.fct.2009.02.018.
Georges, J. et al. (2014) ‘The effects of probiotic supplementation on lean body mass, strength, and power, and health indicators in resistance trained males: a pilot study’, Journal of the International Society of Sports Nutrition, 11(S1), p. P38. doi: 10.1186/1550-2783-11-S1-P38.
Gepner, Y. et al. (2017) ‘Combined effect of Bacillus coagulans GBI-30, 6086 and HMB supplementation on muscle integrity and cytokine response during intense military training’, Journal of Applied Physiology, 123(1), pp. 11–18. doi: 10.1152/japplphysiol.01116.2016.
Hun, L. (2009) ‘Original Research: Bacillus coagulans Significantly Improved Abdominal Pain and Bloating in Patients with IBS’, Postgraduate Medicine, 121(2), pp. 119–124. doi: 10.3810/pgm.2009.03.1984.
Hyronimus, B. et al. (2000) ‘Acid and bile tolerance of spore-forming lactic acid bacteria’, International Journal of Food Microbiology, 61(2–3), pp. 193–197. doi: 10.1016/S0168-1605(00)00366-4.
Jäger, R. et al. (2016) ‘Probiotic Bacillus coagulans GBI-30, 6086 reduces exercise-induced muscle damage and increases recovery’, PeerJ, 4, p. e2276. doi: 10.7717/peerj.2276.
Jäger, R. et al. (2018) ‘Probiotic Bacillus coagulans GBI-30, 6086 Improves Protein Absorption and Utilization’, Probiotics and Antimicrobial Proteins, 10(4), pp. 611–615. doi: 10.1007/s12602-017-9354-y.
Kalman, D. S. et al. (2009) ‘A prospective, randomized, double-blind, placebo-controlled parallel-group dual site trial to evaluate the effects of a Bacillus coagulans-based product on functional intestinal gas symptoms’, BMC Gastroenterology, 9(1). doi: 10.1186/1471-230X-9-85.
Keller, D. et al. (2017) ‘Bacillus coagulans GBI-30, 6086 increases plant protein digestion in a dynamic, computer-controlled in vitro model of the small intestine (TIM-1)’, Beneficial Microbes, 8(3), pp. 491–496. doi: 10.3920/BM2016.0196.
Kimmel, M. et al. (2010) ‘A controlled clinical trial to evaluate the effect of Ganeden BC30 on immunological markers’, Methods and Findings in Experimental and Clinical Pharmacology, 32(2), p. 129. doi: 10.1358/mf.2010.32.2.1423881.
Levers, K. et al. (2015) ‘Powdered tart cherry supplementation surrounding a single bout of intense resistance exercise demonstrates potential attenuation of recovery strength decrement with no definitive oxidative or inflammatory effect’, Journal of the International Society of Sports Nutrition, 12(S1), p. P25. doi: 10.1186/1550-2783-12-S1-P25.
Maathuis, A., Keller, D. and Farmer, S. (2010) ‘Survival and metabolic activity of the GanedenBC 30 strain of Bacillus coagulans in a dynamic in vitro model of the stomach and small intestine’, Beneficial Microbes, 1(1), pp. 31–36. doi: 10.3920/BM2009.0009.
Nyangale, E. P. et al. (2015) ‘Bacillus coagulans GBI-30, 6086 Modulates Faecalibacterium prausnitzii in Older Men and Women’, The Journal of Nutrition, 145(7), pp. 1446–1452. doi: 10.3945/jn.114.199802.
Stecker, R. A. et al. (2020) ‘Bacillus coagulans GBI-30, 6086 improves amino acid absorption from milk protein’, Nutrition & Metabolism, 17(1), p. 93. doi: 10.1186/s12986-020-00515-2.